Fall 2024, Issue 2 - read it below or see a PDF of this issue.
Thanks to you and your family for participating!
Study enrollment continues. We are committed to keeping you updated. In this newsletter issue, you can see the progress we are making together. We gathered this information at the end of July 2024 to share with you.
381 children and adolescents were enrolled in Phase 1.
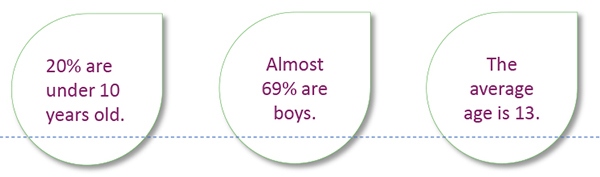
20% are under 10 years old.
Almost 69% are boys.
The average age is 13.
Of the 381 enrolled in Phase 1:
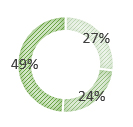
27% have inflammation of the small bowel only.
24% have inflammation of the colon only.
49% have inflammation in both the small bowel and the colon.
156 have entered Phase 2.
That means they have a confirmed diagnosis of Crohn’s disease and are eligible to continue. 144 of 156 Phase 2 participants have started anti-TNF therapy.
In Phase 2: 77% were White, with 11% self-identifying as Black or African American, 8% as another race, 3% identified as more than one race, and 1% not responding or identifying as unknown. 11% identified as Hispanic or Latino.
Of those enrolled:
Almost 23% had a slowdown in their growth prior to diagnosis.
Almost 80% had moderate to severe symptoms at diagnosis.
Our goal is to help put future pediatric patients on a faster path to remission.

You Make the Difference.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. CAMEO (Clinical, endoscopic, and imaging outcomes of children newly diagnosed with Crohn’s disease) is funded by the National Institutes of Health (NIH) through the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Research reported in this publication was supported under Award Number 1U01DK134356-01. cameostudy.org
